Summary
Alternative Name(s): Receiver Power and Data Decoder
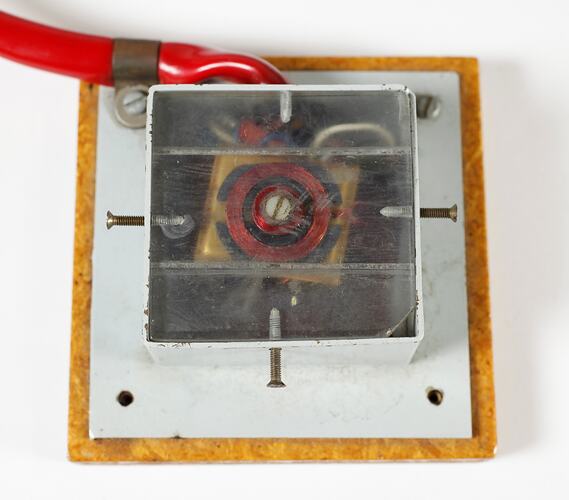
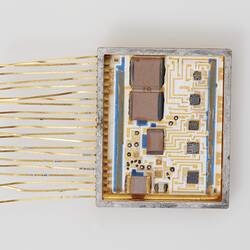
The test jig for transmitting the power and data via the induction coils. The data controlling the patterns of nerve stimulation had to be transmitted through the skin by radio waves as well as the power required to operate the implanted unit. All of the stimulus requirements were realised as a circuit design using transistor components. A radio frequency interface to transmit the information and power through the intact skin rather than ultrasound or infrared light was used as it was more robust and efficient.
This method of transferring power and data avoided the problem of having a permanent connection through the skin, which entailed a risk of infection.
Significance
The bionic ear (also known as the cochlear implant) is the result of pioneering research commenced by Professor Graeme Clark in the late 1960s at the University of Melbourne's Department of Otolaryngology. At the time it was considered that the production of a successful cochlear implant would be extremely difficult making Government funding opportunities almost impossible. Professor Clark and his staff sought donations from the general public to originally get the project off the ground. Assistance from clubs such as Rotary, Lions and Apex was invaluable at this time.
The prototype multiple-electrode bionic ear was implanted in the first adult at The Royal Victorian Eye and Ear Hospital by Graeme Clark and colleagues in 1978. The team discovered a way to analyse complex speech signals and present it to the auditory nerve as electrical stimulation in a way that could be deciphered. They were able to engineer a speech processor small enough for a patient to wear.
As a result, the Australian Government awarded a public interest grant that helped develop the bionic ear industrially by the Australian firm Cochlear Limited. The first device for clinical trial world-wide was implanted at the Royal Victorian Eye and Ear Hospital in 1982. The international trial established that it was safe and effective and it was approved by the US Food and Drug Administration in 1985, the first multiple-electrode bionic ear to be approved by any world regulatory body.
In 1985, the team implanted the first child with a multiple-electrode bionic ear. It was developed and manufactured by Cochlear Limited in co-operation with The University of Melbourne and The Bionic Ear Institute. This was the start of a world-wide trial for the bionic ear in young children. It was approved as safe and effective for use in children born deaf or developing hearing early in life by the US Food and Drug Administration in 1990. It has also been approved by other world regulatory bodies. It is considered by many the first major advance in helping profoundly deaf children to communicate in the last 200 years since signing was established at the Paris Deaf School. The Australian Bionic Ear has now been implanted in more than 50,000 people in more than 120 countries.
Research work on the bionic ear requires collaboration between many fields. They include auditory neurobiology, auditory physiology and perception, bionics programming, psychology and neuro-engineering. In developing the bionic ear, much has been learned about nanotechnology and biotechnology including: tolerance of surgical implants by the body; interfacing electronic devices with nerves and the brain; improving speech understanding and language in deaf people; and repairing nerves. Today the knowledge of biomaterials is not only applied to hearing, but also to tackling spinal injury and epilepsy.
Graeme Clark has lodged some artefacts with the National Museum of Australia. He has also deposited material with the National Film and Sound Archive.
More Information
-
Collection Names
-
Collecting Areas
-
Acquisition Information
Transfer from University of Melbourne (The), Cochlear Pty Ltd, 12 Nov 2008
-
Lead Researcher
Professor Graeme M. Clark AC - University of Melbourne (The), 1976
-
Classification
-
Category
-
Discipline
-
Type of item
-
Keywords
Australian Innovations, Disabilities, Disability Technologies, Hearing Aids, Hearing Impaired, Innovation & Design